pH STABILTY (POTENTIAL HYDROGEN)pH is a major factor in the ability of the plant to absorb all the necessary macro and micro elements needed for sugar production. pH is the term used in measuring the acidic, (lower level), or alkalinity, (upper level) of the nutrient solution. pH levels in your town water should be between 6 to 7. The ideal level for growing crops in hydroponics is 6.3 ph. At this level the plant has access to all 24 mineral salts needed for strong healthy growth. The ph is read on a scale of 0 to 14. In a soil or organic situation the ph can vary dramatically within just a a few square centimetre, and can be difficult to bring back into balance, however in hydroponics we use inert materials as the support structure of the plant so it is quite simple to adjust the ph at any given time. If your water supply is reading below 6.0 pH or above 7.0 pH then you have other chemical influences which could effect your crop's growth. A water purifier would be a good investment at this time, as this is the only way you are going to get a stable (neutral) pH, the other alternative is to forget about growing at the current site or relocate. If your pH in the tank falls below 6.0 pH it can lock out valuable macro elements like phosphorous, calcium, nitrogen and magnesium. If it goes above say 7pH (alkaline), micro elements such as iron, boron, manganese and zinc are locked out. This will show up as a deficiency in the plant, which is extremly difficult to rectify after the fact. The most common way of treating this deficiency is to foliar spray the crop with the element it is deficient in. Knowing which mineral is lacking is where the difficulty lies! Without the proper facility to identify the mineral lacking in the plant it is almost impossible to fix the problem. The only way to test for mineral deficiencies is by way of leaf tissue analysis. This can be a costly exercise and often takes 7 to 10 days to get results back and by that stage it is just about too late for the grower to rectify the problem. I would suggest if you have a situation whereby your ph levels drop or increase dramatically, is to install a float valve and a water backup tank which at least will help in the buffering the ph slightly. If you wish to grow crops of any kind successfully you will need to get some sort of daily maintenance routine in place to check pH levels as well as nutrient concentrations so as to avoid the above problems of dramatic shifts in pH. If monitoring the pH with a digital pH meter, make sure they are calibrated at least once a week, if not you must dump your tanks weekly. Dumping tanks weekly is the only way you are going to keep the ph stable. When you ad pH adjusters make sure you dilute the concentrate as it is heavier than the water molecule and will sink to the bottom, if the solution turns milky white in the tank you have made it too strong, the milky white substance is the acid coming in contact with the elements and precipitating them out, which means it is stopping the plant from being able to take elements in. If you find this has happened it is always best to dump the tank and re-dose with fresh water and nutrient. How to calibrate a pH meter
These instructions refer specifically to the Blue Lab range of meters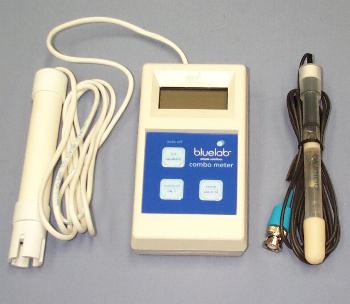
  STEP 1:Thoroughly wash the pH probe with clean water, shake off excess water and place in buffer solution pH7 first. Allow the meter reading to settle for a minute and then when it has, press and hold the button marked pH/Calibrate until the the "CAL" sign shows in the display.
STEP 2:Thoroughly wash the probe again with clean water and then repeat STEP 1, but this time use the pH4 buffer solution. Your meter is now reading the pH accurately!
|